 Heart rhythm disorders are common and may have devastating consequences. The group for translational electrophysiology - a collaboration of the Department of Cardiology, Bern University Hospital, and the ARTORG Cardiovascular Engineering group - aims at developing tools and devices for cardiac rhythm management.
Heart rhythm disorders are common and may have devastating consequences. The group for translational electrophysiology - a collaboration of the Department of Cardiology, Bern University Hospital, and the ARTORG Cardiovascular Engineering group - aims at developing tools and devices for cardiac rhythm management.
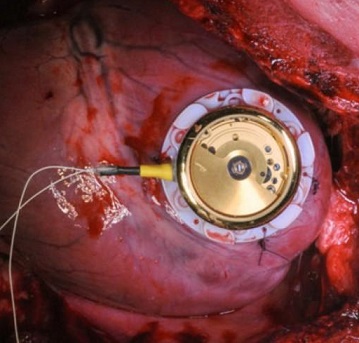
A main research focus is the development of novel technologies for cardiac pacing. Contemporary pacemakers suffer from limitations. Pacing leads are prone to dislocations and isolation defects. Recently introduced leadless pacemakers overcome this limitations, however, do not allow for dual-chamber pacing. We are implementing ultra low-power communication technology in custom-built leadless pacemakers to allow for multisite pacing. Another limitation of today's pacemakers is their limited longevity due to exhausted batteries. We are looking into methods for intracorporeal energy scavenging which would allow designing lead- and batteryless pacemakers in future.
A second major research area of our group is the development of tools for arrhythmia diagnosis. Electrophysiologic examinations provide detailed informations but are invasive, time-consuming and costly. We are working on novel minimal-invasive alternatives to perform precise bedside EP examinations.
Main contact: Andreas Haeberlin, MD, PhD